1. What Is Silica—and How Does It Get Into Your Water?
Silica (SiO₂), or silicon dioxide, is one of the most abundant mineral compounds on Earth—commonly present as quartz or silicate minerals. As groundwater moves through rock, small amounts of silica dissolve into the water. While silica’s solubility is relatively low, detectable levels are common in many supplies.
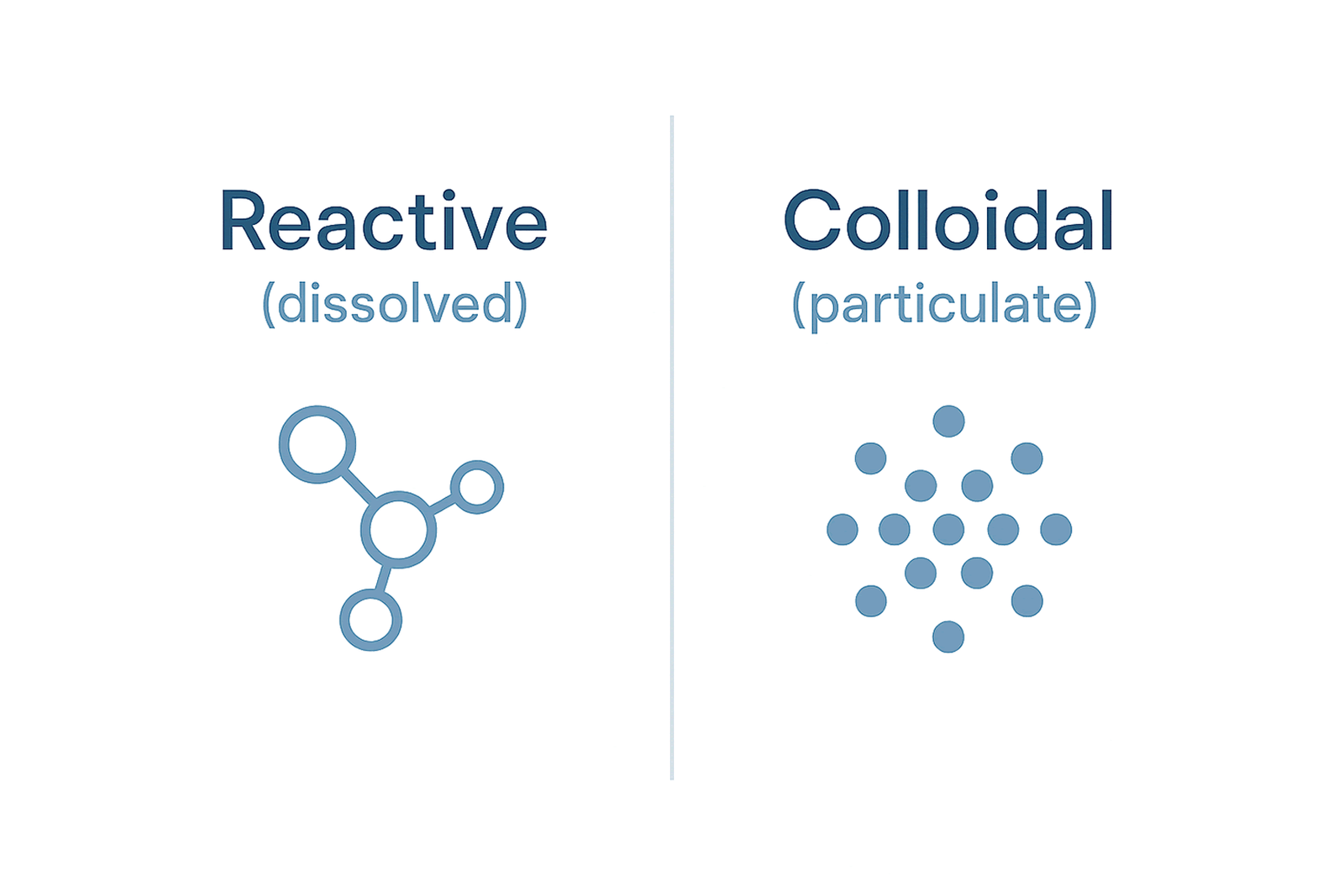
Silica in water typically exists in two forms:
- Reactive (monomeric/bisilicate) silica: Dissolved and weakly acidic; can be removed by strong-base anion exchange resins—generally industrial-scale.
- Colloidal (non‑reactive) silica: Microscopic polymeric particles that resist conventional filtration and ion exchange.
2. When Does Silica Become a Problem?
At concentrations above roughly 20–25 mg/L, silica may cause noticeable household issues, including:
- Etched or cloudy glassware, especially with aggressive dishwasher detergents. Etching often begins as a thin film and can progress to permanent damage.
- Stubborn deposits that resist regular cleaners:
- Mineral buildup at the toilet waterline (may require pumice or careful scraping).
- White “islands” or filmy deposits on black sinks, chrome fixtures, and shower doors.
- White haze on granite counters.
- Mostly aesthetic, not health risks: There is no strong evidence that ingesting silica at typical household levels is harmful.


3. How to Remove Silica from Water—What’s Feasible for Homes?
Many industrial silica‑treatment methods are not practical at home. Here’s a realistic look for residential use:
| Method | Removes Silica Type | Residential Suitability | Notes |
|---|---|---|---|
| Lime–Soda Softening | Reactive & Colloidal | Not practical | Complex chemistry and sludge handling; industrial‑scale process. |
| Ultrafiltration (UF) | Colloidal | Possibly | Effective for colloids; does not remove reactive (dissolved) silica. |
| Ion Exchange (Anion Resins) | Reactive | Not practical | Requires hazardous regenerants (e.g., NaOH) and careful operation. |
| Reverse Osmosis (RO) | Both | Practical (point‑of‑use) | Whole‑house RO possible but costly; typically best for drinking water. |
| Electrocoagulation / EDI | Both | Not practical | Primarily industrial or emerging technologies. |
4. Useful Solutions (and Practical Tips)
- Whole‑house RO (properly sized and pre‑treated) can reduce both reactive and colloidal silica, but it requires significant investment and maintenance.
- Ultrafiltration may help with colloidal silica but offers no relief for reactive forms.
- Community‑tested guidance:
- UF membranes around ~0.01 µm can help for colloids.
- Anion systems for reactive silica are often expensive and maintenance‑heavy due to chemical handling.
5. How to Manage Silica Build‑Up at Home
- Lower dishwasher water temperatures (< 140 °F).
- Use soft‑water, phosphate‑free detergents; consider air‑drying glassware.
- Hand‑wash fine glassware to prevent etching.
- After showers, wipe down chrome and glass with a squeegee.
- For tough deposits, use silica‑targeted cleaners and careful mechanical removal (light scraping) as needed.
- Apply protective glass/surface coatings to reduce future buildup.
6. Final Thoughts
- Silica in water is generally an aesthetic nuisance—not a health threat at household levels.
- Practical residential options: RO for drinking water; UF only for colloidal issues. Most other methods are industrial‑grade and impractical at home.
- Prevention and maintenance are often more cost‑effective than attempting whole‑home removal.
